Before a COVID-19 vaccine can become a household name, it goes through months of testing by researchers around the world. Here in Halifax, scientists at the Canadian Centre for Vaccinology are working on three made-in-Canada vaccines—Medicago, VIDO and VBI—in hopes of finding the next Moderna, Pfizer and AstraZeneca. And they are looking for human guinea pigs who want to be test subjects.
“Right now, we’re doing the first clinical trials of new COVID vaccines, featuring those that have been developed, that are based on Canadian technologies,” says Scott Halperin, an infectious disease specialist involved with the research. These vaccines are all created by Canadian companies that received federal funding: Medicago in Montreal, VIDO in Saskatchewan and VBI in Ottawa.
To get to the clinical trial stage, candidate vaccines go through “pre-clinical laboratory evaluations and are tested in animals,” Halperin says, “then they go to Phase 1 studies.” These are the smallest, using from a few dozen up to about 100 human volunteers. Halperin says the Halifax studies are using about 70 people.
“People who might not be in line to get a vaccine ‘til June or July, those are the people we’re looking for.”
tweet this
“It’s first tested in young, healthy people, so people who are in the age range of 18-40 or 18-50 years old,” says Halperin. After that, the range is slowly expanded to older individuals.
“We go from the least vulnerable to the most vulnerable people first, to make sure that it’s safe for people who have very low risks of having underlying medical conditions and that sort of thing. And then we gradually expand it, so that when it’s finally authorized for use at the very end of the day, it’s been tested in people of all sorts.”
Of the three Canadian vaccines, Halperin says right now VIDO is in the middle of Phase 1 testing, and VBI is about to move on to Phase 2, which will see about 700 or 800 people test the vaccine. “Whereas the Phase 1 studies were totally healthy people, the Phase 2 studies might be in people who have underlying conditions but are reasonably stable. So if they have high blood pressure, it’s well controlled. If they have diabetes, it’s well controlled. You’re getting people who are more like the general population.”
Throughout the process, Halperin says the volunteers are under close watch for potential side effects.
“We are monitoring extremely carefully. People are getting a lot of tests done when they first come into the study, and then they’re monitored very closely through diaries for the first month afterwards,” he tells The Coast in a phone call. “And then they are in touch with us”—at the vaccinology centre—“for any adverse event they have, whether they think it’s related to the vaccine or not.”
If all looks good in Phase 2 and the adverse events are minimal, vaccines move on to Phase 3, which is where Montreal-made Medicago is heading next. “Most of the Phase 3 studies for COVID have been a range of 30 to 40 thousand people,” says Halperin.
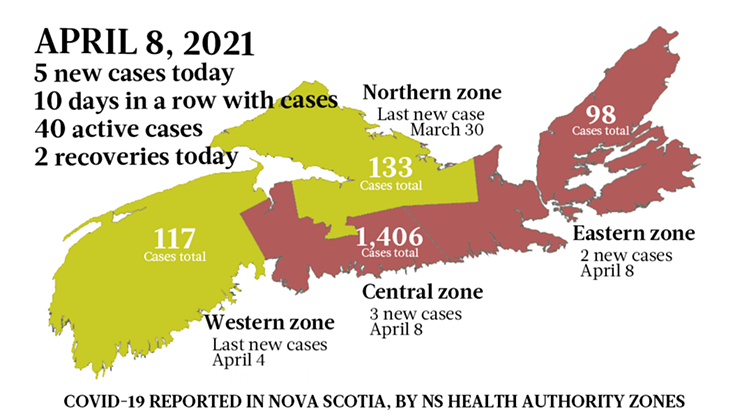
These Phase 3 studies actually can’t be done in Canada, much less the Maritimes, because of how low our case numbers are. “Most of the Phase 3 studies for Moderna and for Pfizer, they were done in the United States back when the United States was a disaster, or they were done in South Africa or Pakistan or South America where there are a lot of cases right now,” Halperin says.
Passing Phase 3 is the goal for a candidate vaccine. After that, it can go to Health Canada and/or other national agencies around the world, to be approved for use country by country. Halperin seems hopeful that of the three Canadian vaccines, at least one will make it to the public. But if there are major effects, or if minor side effects like arm pain appear in a majority of the population, or if the vaccine just isn’t effective in humans, it’s back to the drawing board.
“Some of them won’t succeed, but obviously no vaccine can succeed without starting. So they have to start these trials, it’s the process that has to be gone through, and we’re hopeful that many of them will succeed,” Halperin says. “Because even with all the vaccines we’ve heard about that are now authorized, there’s not enough capacity to immunize the world. So we need more vaccines, different types, in order to have enough vaccine to immunize everybody in the world who wants a vaccine.”
The Canadian Centre for Vaccinology has an open call for healthy adults on its website. Participants must be available to go to 11 appointments over the next 12 months and meet a few other basic qualifications, but Halperin says the centre is always happy to have new volunteers.
“People very much like to take part in clinical trials. Some people say, ‘No, no way,’ but others say, ‘Sign me up, I want to help.’”
Most of the people he expects to sign up are younger adults who won’t be eligible for other vaccines until later this year. “Obviously if somebody’s eligible for the provincial program where they can get an approved vaccine, that’s the route they should go. But people who might not be in line to get a vaccine ‘til June or July, those are the people we’re looking for.”